New features in TrialGrid (January 2021)
Batch Labelling
TrialGrid allows users to define Labels which can be used to signify workflow state of objects. Labels can be applied to all study design objects so, for example, you can create a label "Ready for Testing" and apply it to Edit Checks and to Derivations. Filters in object listings allow you to find all the objects which have a particular label.
Our last act of 2020 was to release new functionality which allows you to select a set of objects and apply (or remove) labels from them as a batch.
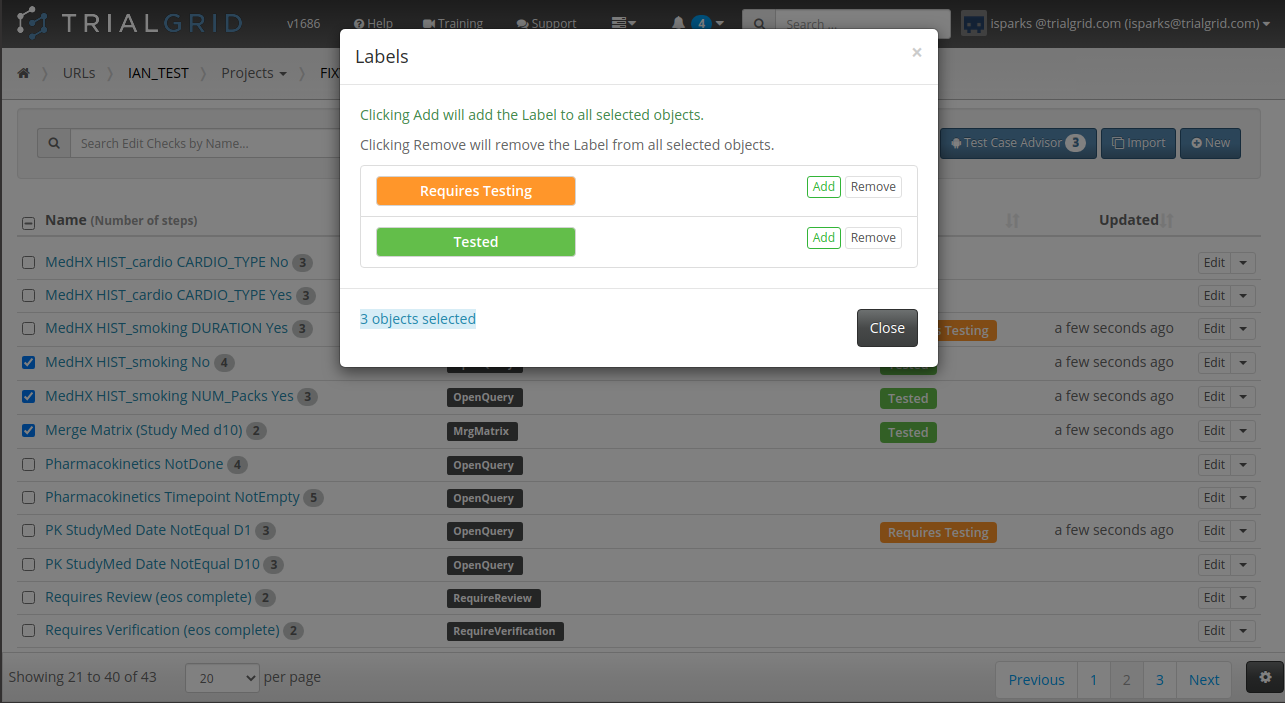
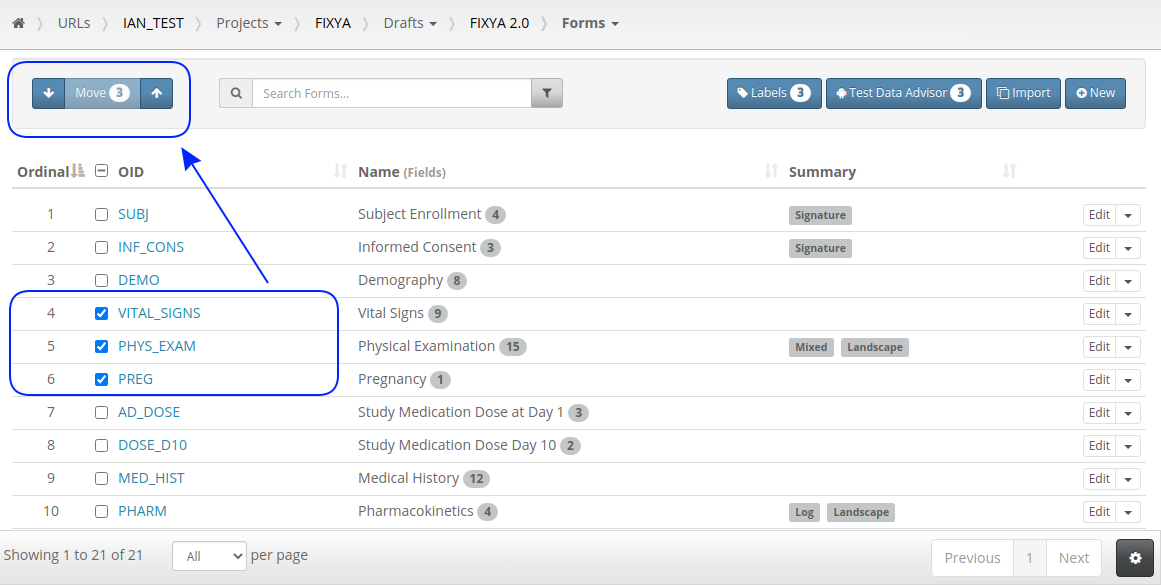
The ability to label objects in bulk is a great feature and the checkbox select on every row has already been used to make Form and Folder re-ordering more intuitive. Just check the rows you want to move and use the up/down buttons:
Improved Annotates
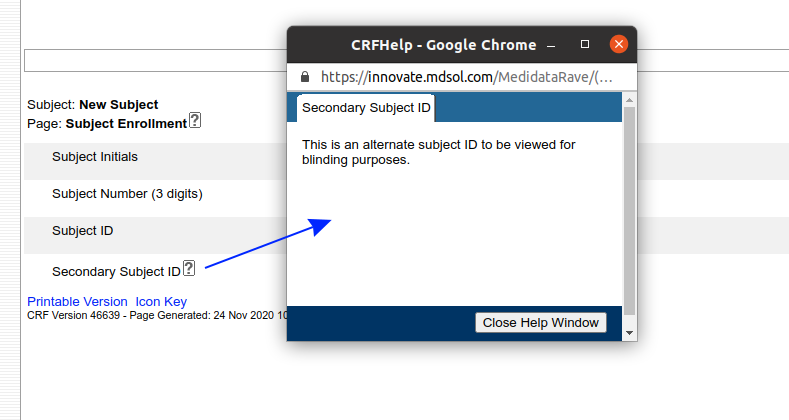
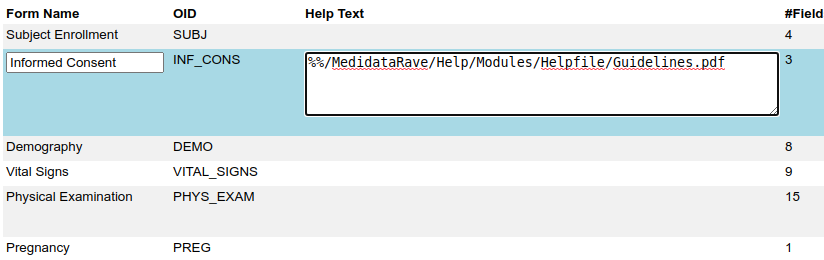
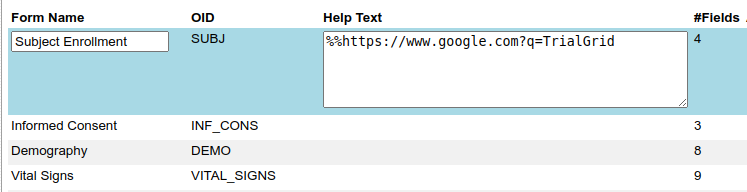
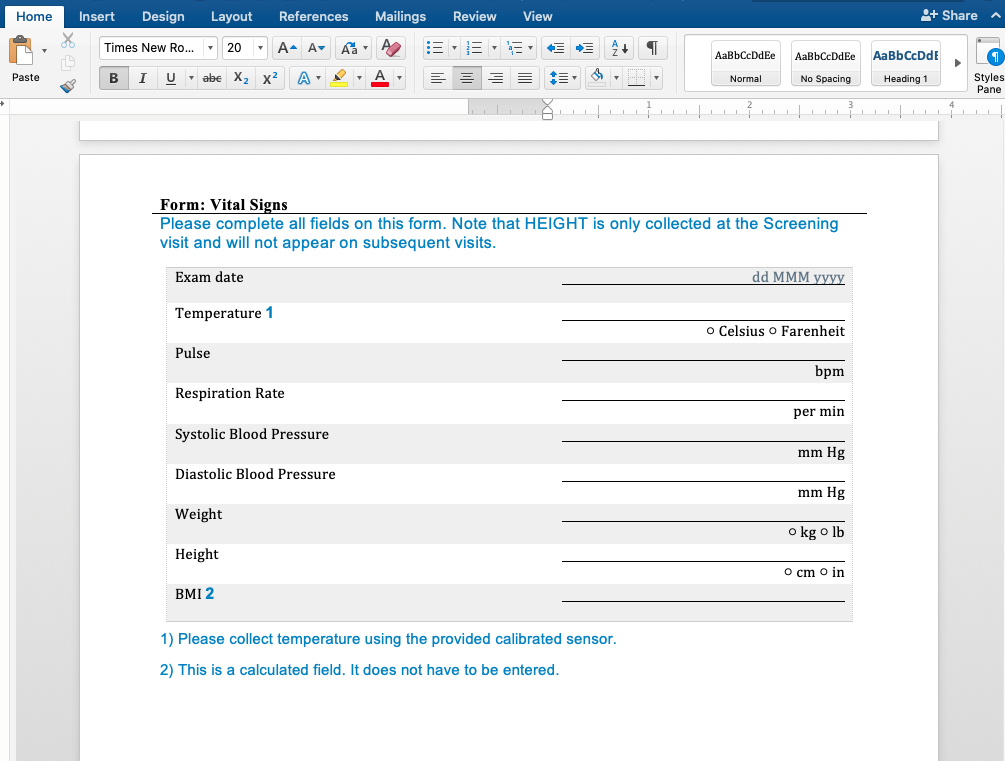
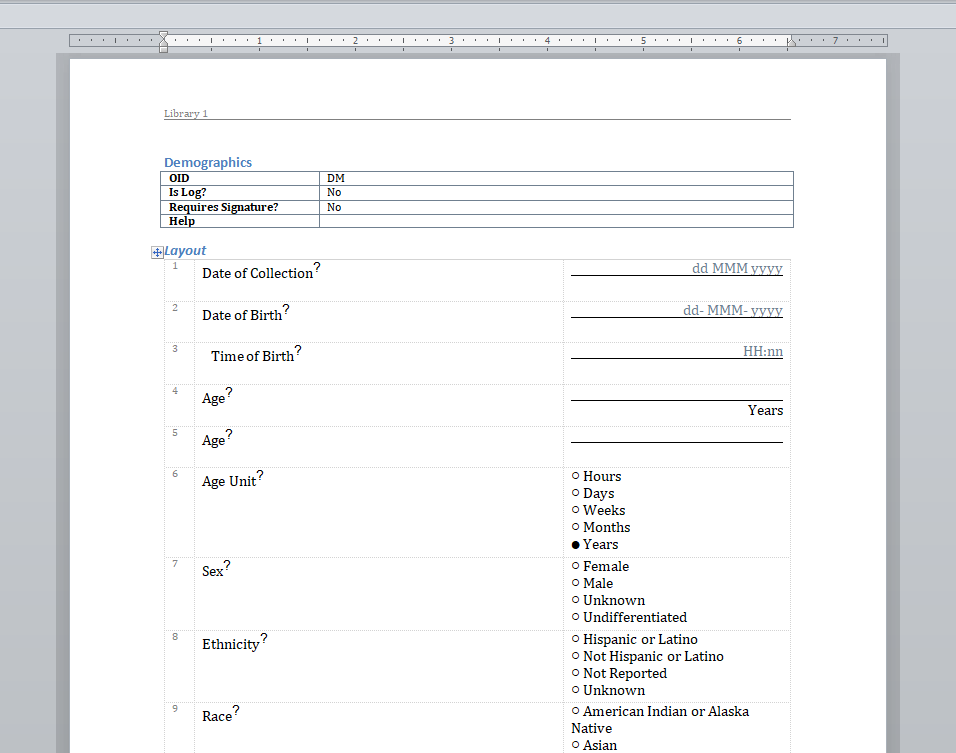
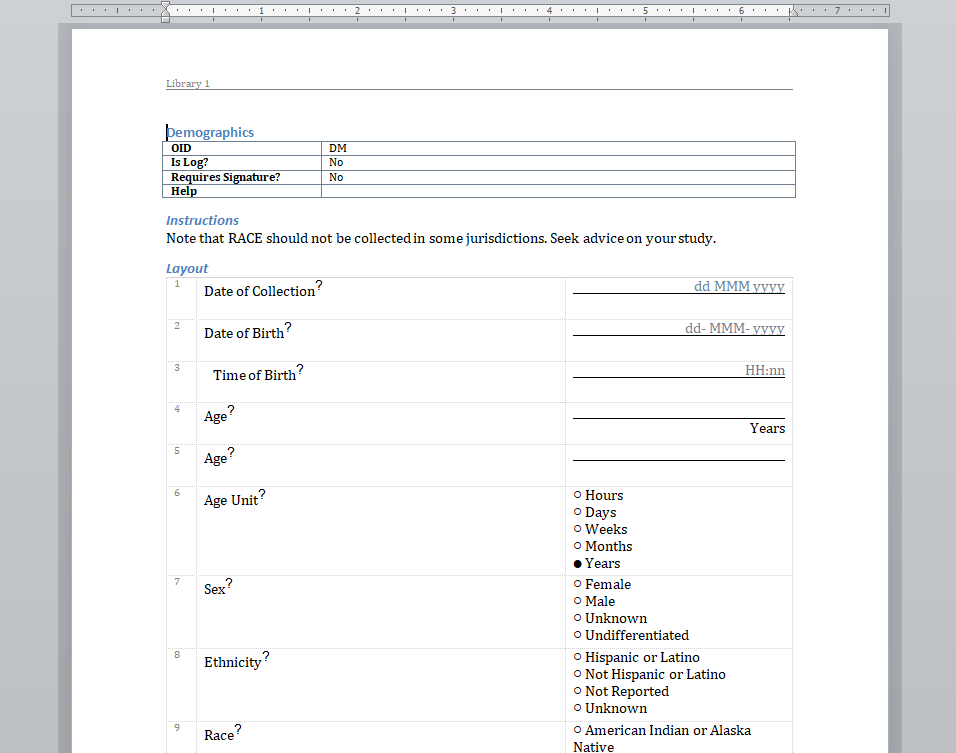
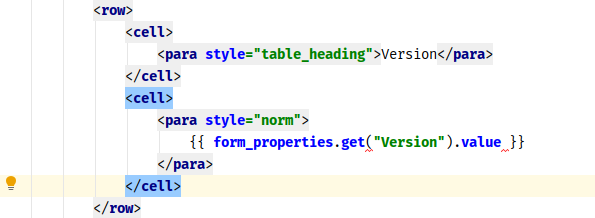
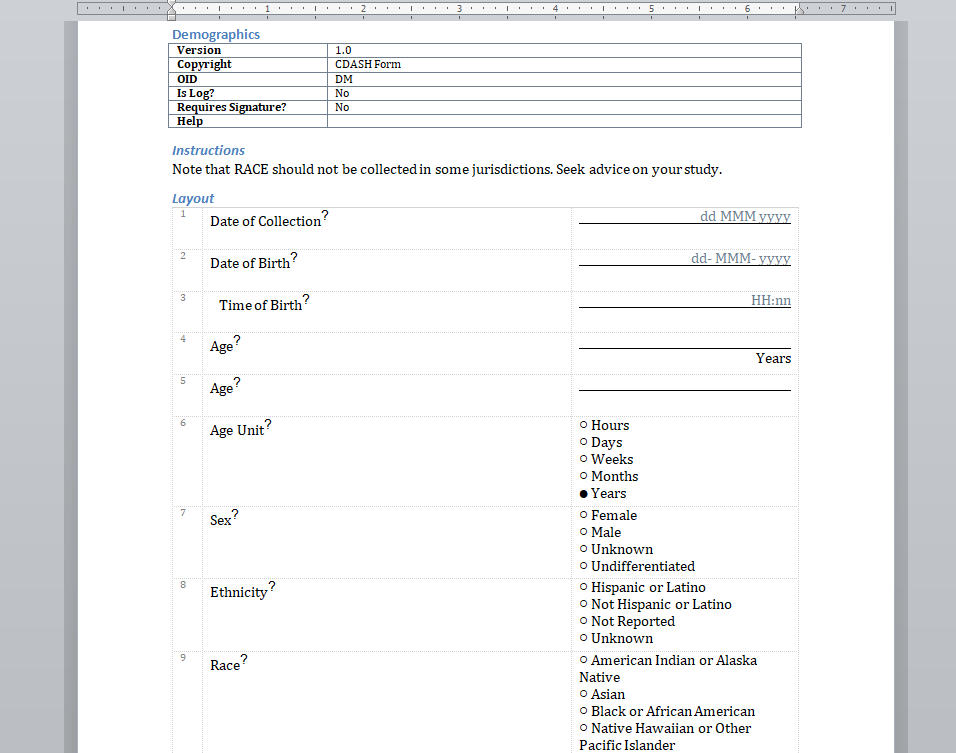
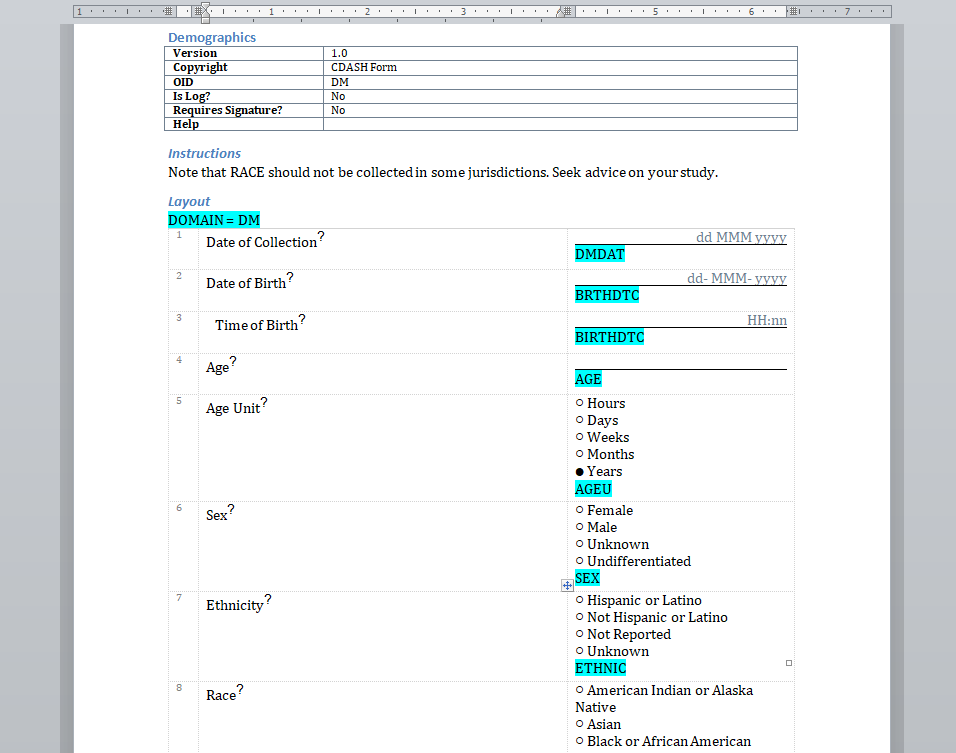
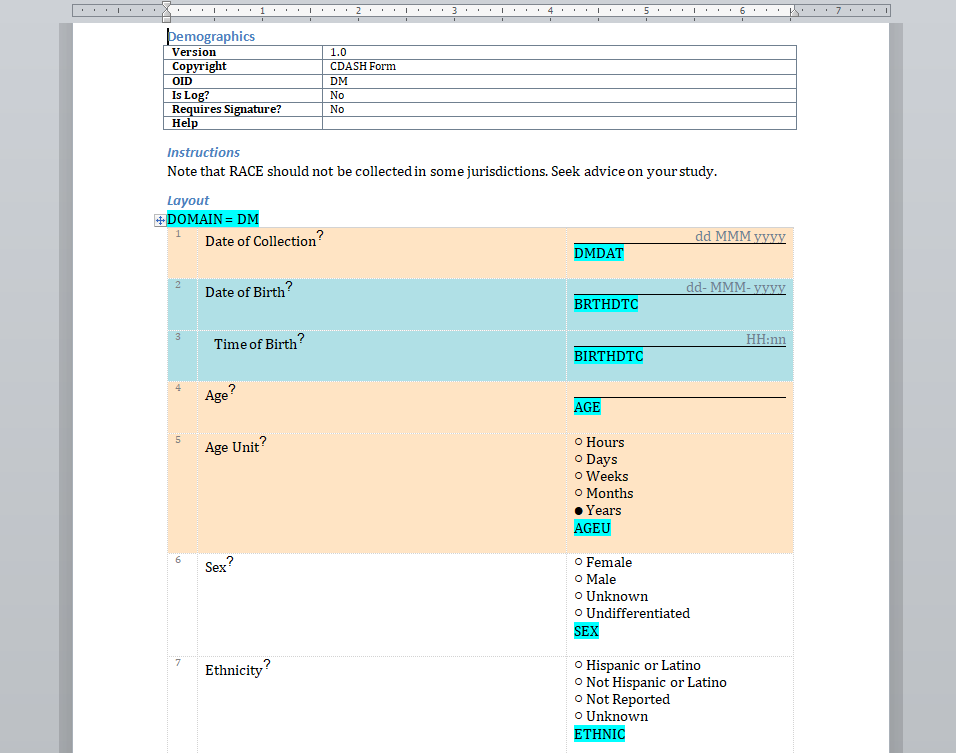
We have written before about our Microsoft-Word based document generation. This is a template driven system that can be used to generate all kinds of documents in Microsoft Word format. We provide some example templates and our latest Annotate template now calls out log sections as you can see in the example below.
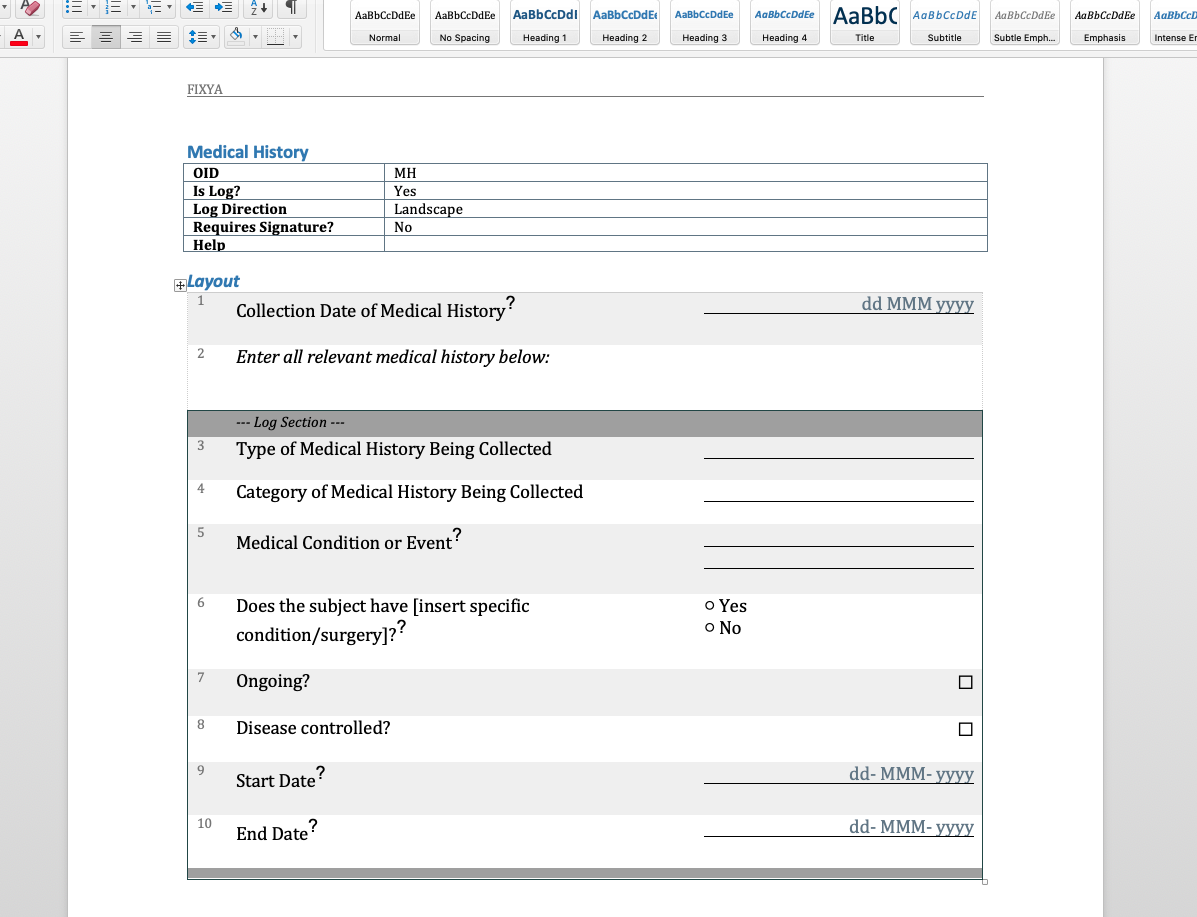
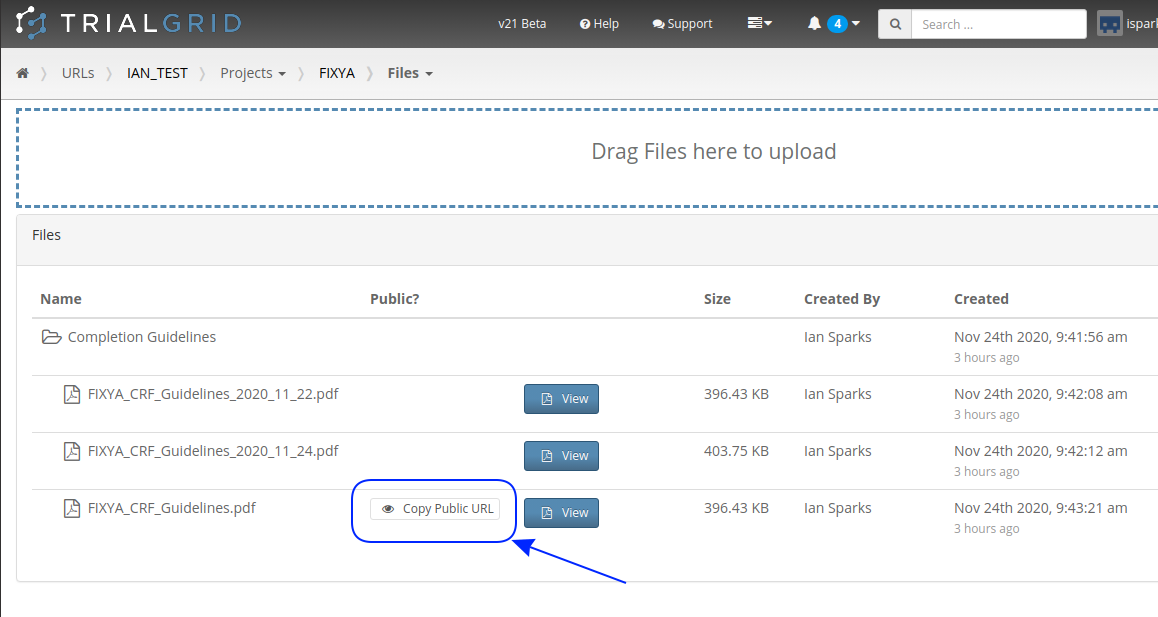
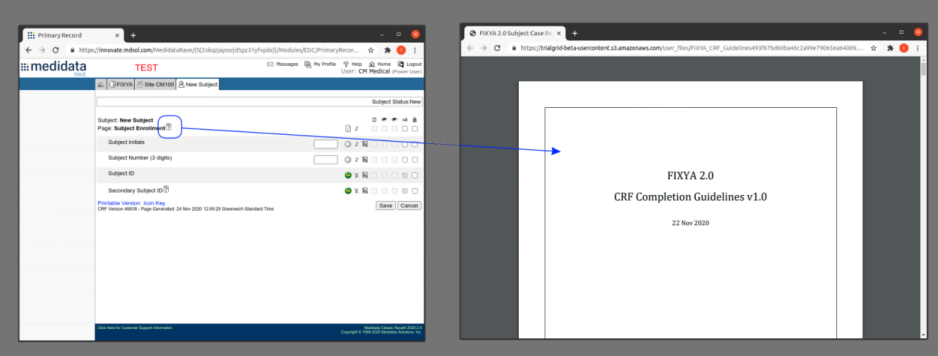
There is a lot more we can do with Word document generation and a few weeks ago we also added PDF generation for customers who want the added security of PDF files. Contact us if you have a need to generate documents from Rave study designs. Whether its annotates, CRF completion guidelines, standard library documentation/usage guides, checklists, object level metrics or something else we can help.
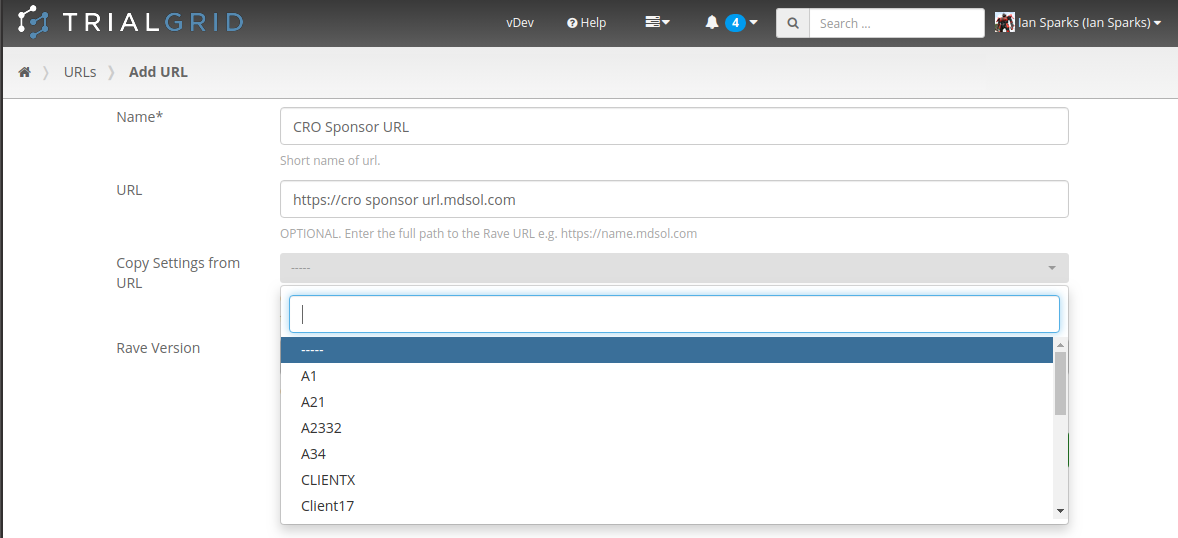
Create URLs
CRO customers in particular will welcome the ability to create their own URL records in TrialGrid. CRO's routinely have Rave URLs which they share with a Sponsor and want a way in TrialGrid to mirror this arrangement. Users can now be assigned to have "Create URL" permission, copying setup such as Annotate Definitions, Labels and Core Configuration from existing URLs if needed.
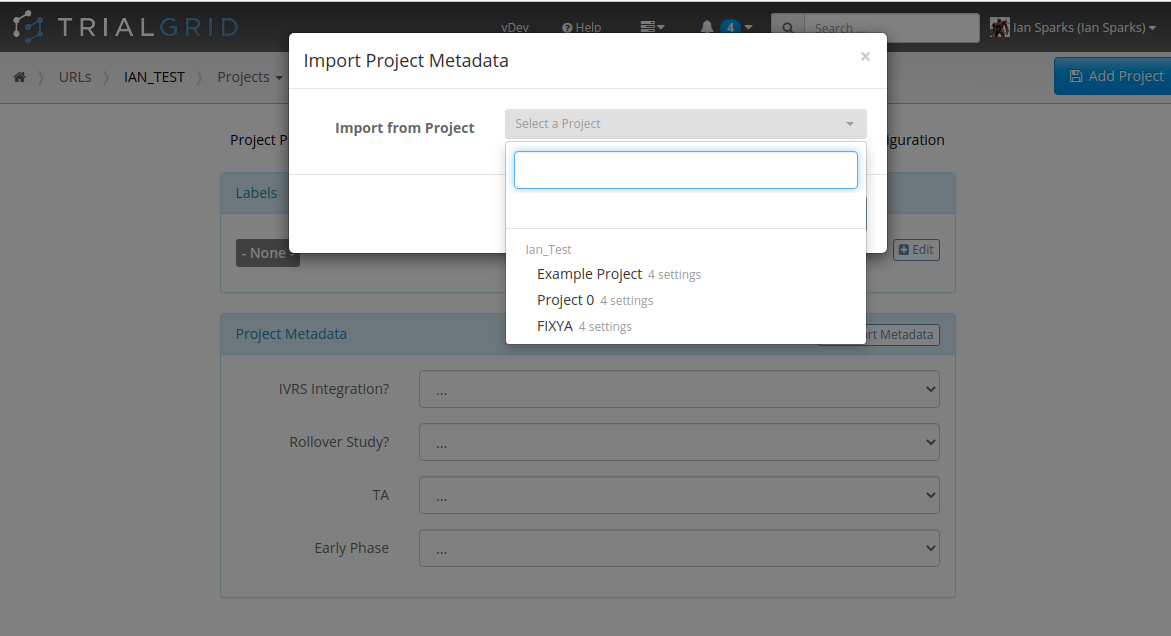
Copy Project Metadata
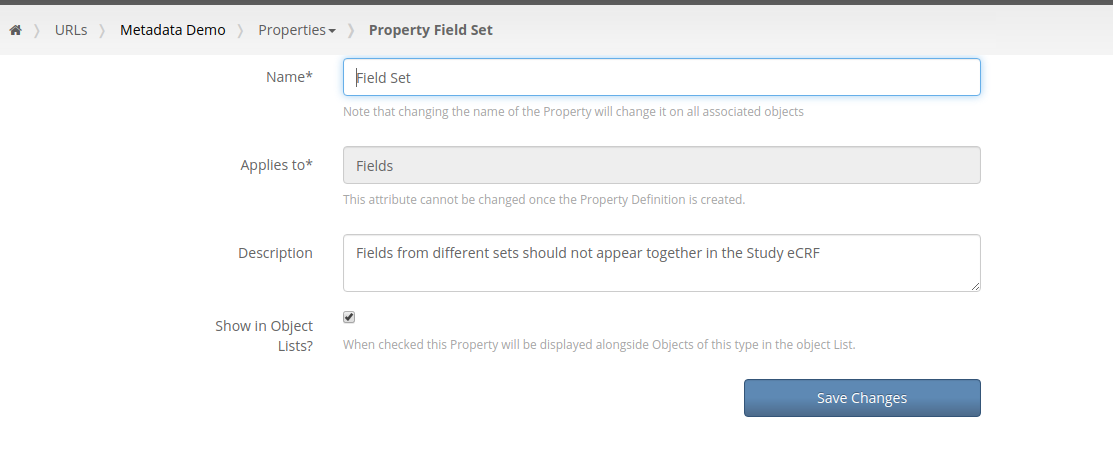
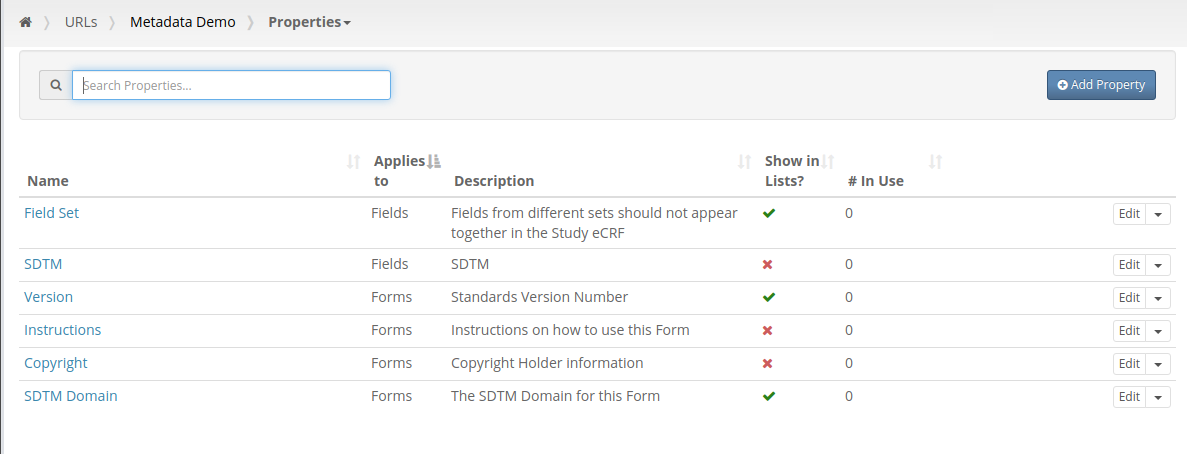
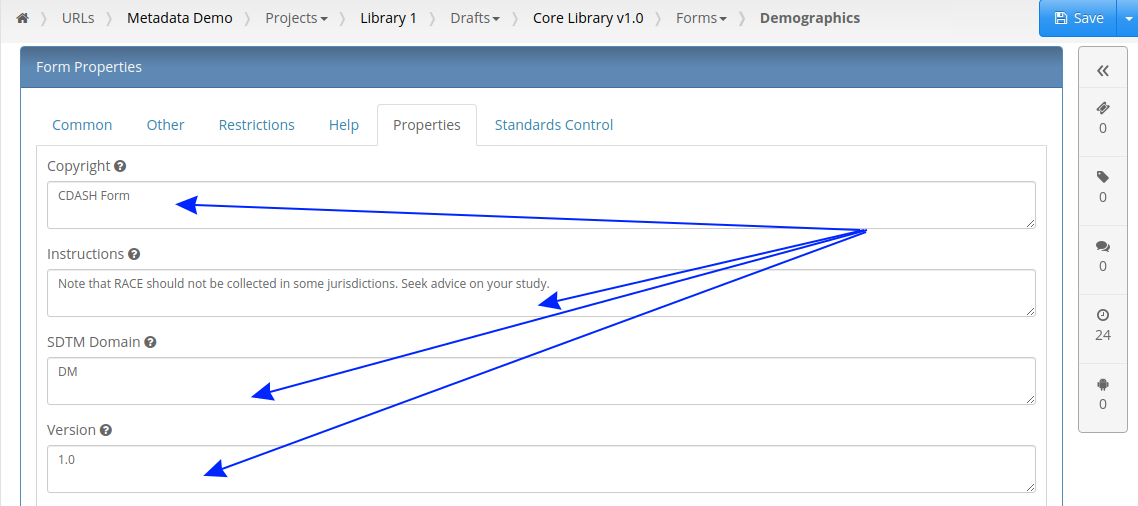
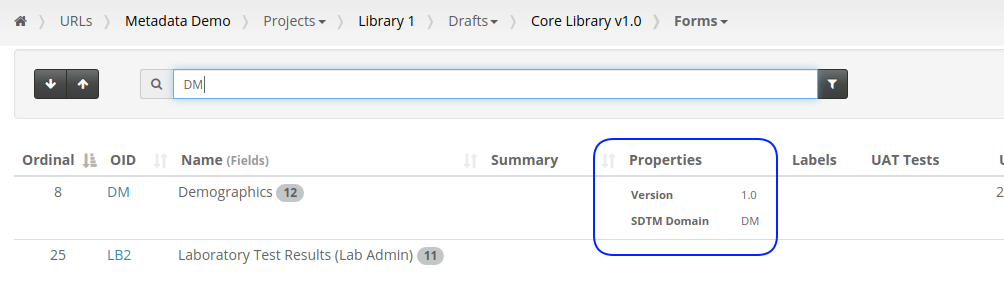
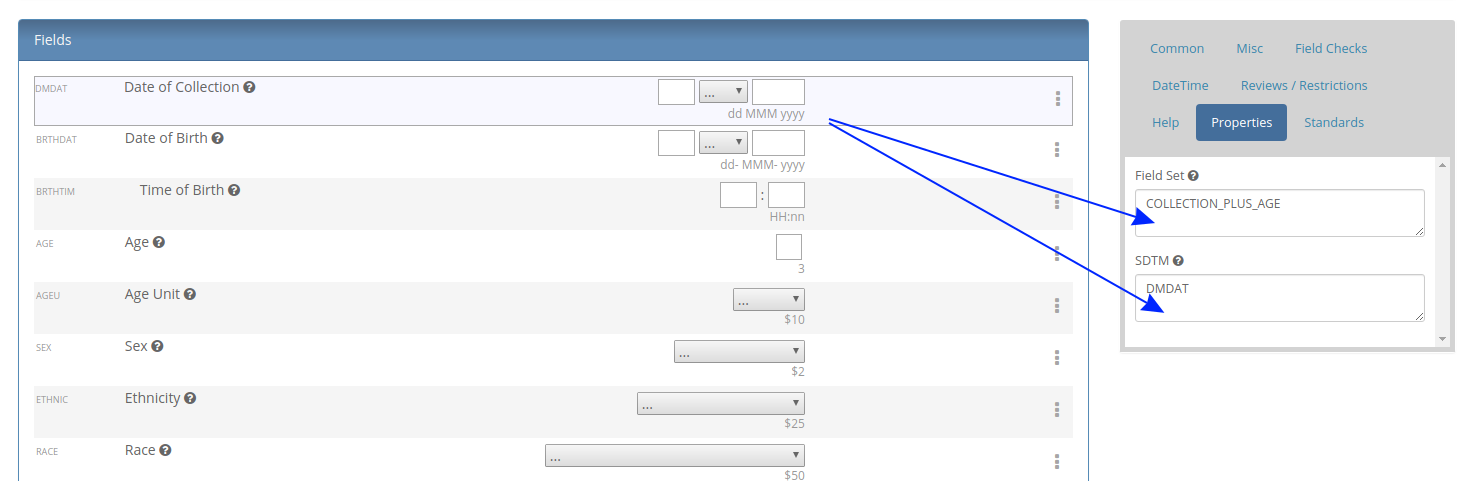
Our first feature of 2021 is a simple way to copy Metadata between Projects. Similar to Labels, TrialGrid allows users to create new Custom Properties (or Metadata) for objects. This includes Projects and Drafts as well as Forms, Folders, Edit Checks, Data Dictionaries etc. For a Project you might use this Metadata to document what Therapeutic Area the Project study is targeting, the type of blinding, the study phase, whether it is a rollover study etc. Custom metadata can appear in project listings and in generated Annotates and it can also be used to drive Standards Compliance Rules (a topic for another day).
When you have many of these Project metadata values, creating a new project and setting all the values can be time-consuming. This feature makes it easier to copy these settings from an existing project.
Summary
We founded TrialGrid in 2016 to bring Medidata customers a better Rave Study build experience. Four years later we've expanded way beyond our initial ideas of Build Quality checks (currently 120 checks) and more intuitive Edit Check building into:
- Automated Testing of Edit Checks and Derivations
- Automated Form Data Entry
- Advanced editors for all Medidata Rave study build objects
- Study build Standards compliance checking and reporting
- Word and PDF document/annotate generation
- Team workflow and collaboration
- Study design visualization tools
- And a lot more
But the idea is the same: better tools for Rave Study build.
We have a lot more to do and most of our new features are driven by customers asking to do more with the TrialGrid system. Watch this space for developments or sign up for our newsletter at the top right of this page. Good luck with your Medidata Rave study build activities in 2021!