CRF Completion Guidelines
What are CRF Completion guidelines?
When a clinical site is recruited to participate in a clinical trial it is common to provide CRF Completion Guidelines - a document which describes the particular data collection requirements for the study. For a study using Medidata Rave this might include guidance on how to log into Rave and navigate the system to perform particular tasks. Typically the guide will be study-specific to familiarize clinical site personnel with key data collection forms and requirements of the protocol.
Support in Medidata Rave
Medidata Rave has a few features which help study builders develop and distribute CRF Completion Guidelines.
First, Rave has an Annotate (or blank Forms) option in its PDF generator which can be useful for generating a visual representation of the forms. Unfortunately the generator does not have an option to include help text entered for forms and fields built into the study design and help text is key information for guidelines. The generator also only outputs PDF files which limits its flexibility because editing PDF files requires special software. The Rave PDF generator can still be a useful starting point and some organizations use it as the basis for their guidelines - adding back the help text and inserting additional pages of information.
The second useful feature is the Rave eCRF itself. Help text entered for forms and fields can be displayed by clicking the help icon next to a field:
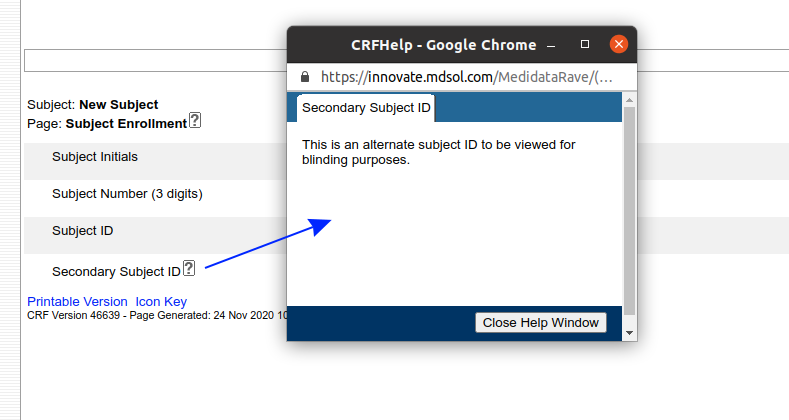
But Rave has another trick. The Field and Form help can be turned into a hyperlink that loads another web page or a document in a new window. By prefixing the help text with %% (two percent signs) you can make the help text link a link to a file hosted by Rave:
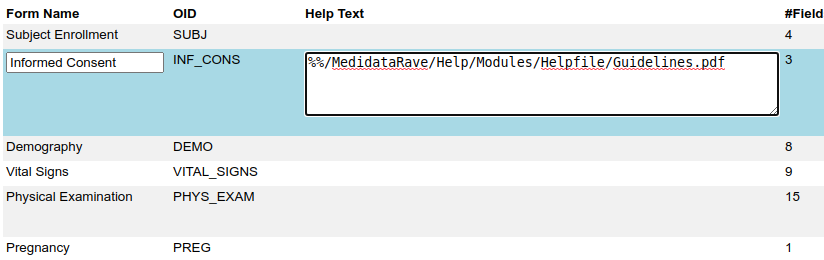
Or to a file or webpage hosted on some other system.
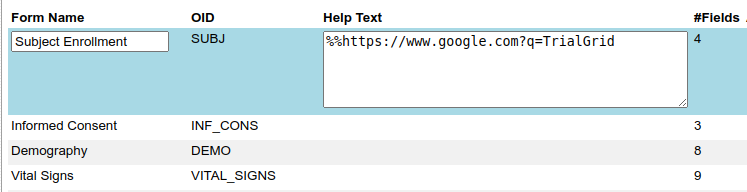
Here we just link to the Google search page but it could be a link to a document to be loaded in the browser.
Support from Medidata
This feature of Rave makes it easy to create a link from field or form help in Rave to a CRF Completion Guidelines file in PDF format. Clicking the link will open the Guidelines in a new browser window.
There isn't a self-service option in Rave for loading CRF Completion Guidelines (or any other type of file) but customers can request Medidata to load files into their Rave instance for them. This is a good option for study build teams which don't have IT support for hosting files but it does not provide the team with much control for updating the Guidelines.
Since you can host these files externally there are many other options. You could use a general file hosting service such as Dropbox but often there are restrictions on the services which a study team can use.
How TrialGrid can help
TrialGrid provides several features that can help study teams with their CRF completion guidelines.
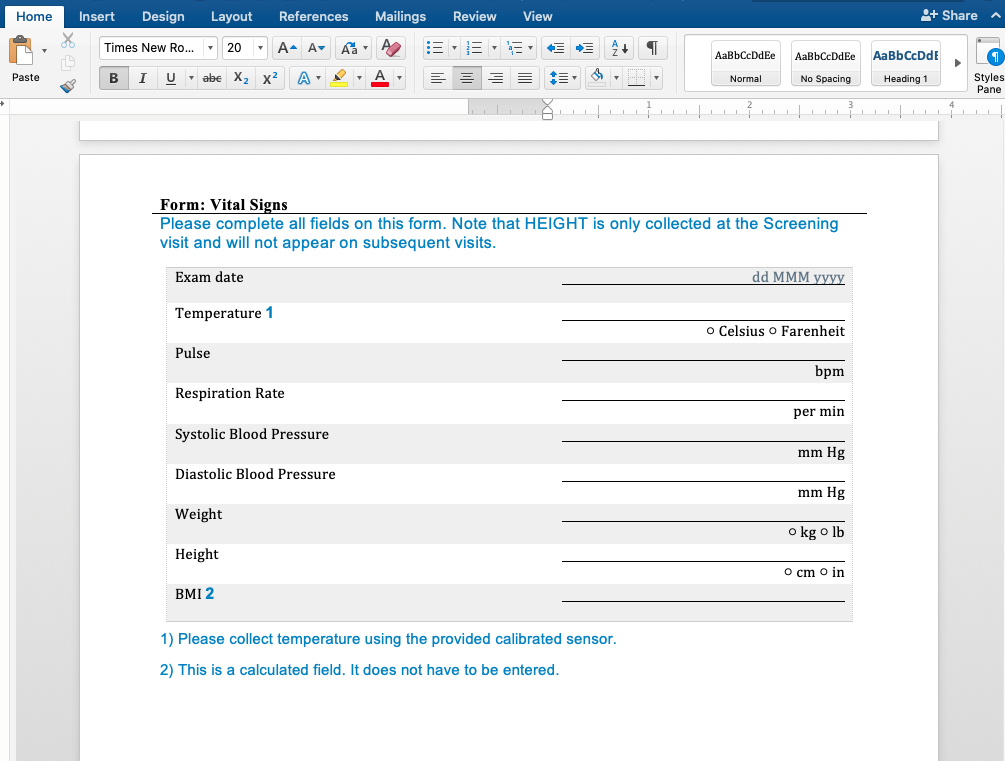
First, our document generation system can generate Microsoft Word as well as PDF documents. Word is a great basis for CRF Completion guidelines because it's easy to insert additional content. Here is a simple example showing form and field help using a document template we developed for a customer. This page was completely generated by our document generator:
Secondly, TrialGrid projects have a files area where study teams can upload files related to their project. Normally these files are private, only team members can download or view them but last month we added a new feature that allows these files to be made public. A file that has been made public can be viewed by anyone with the link.
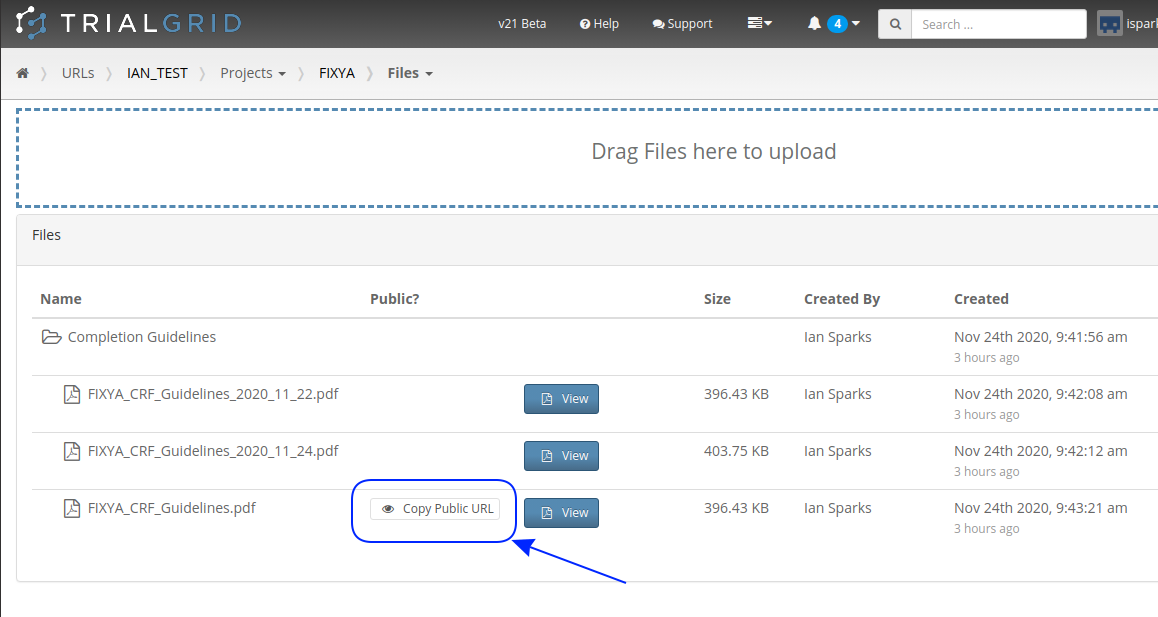
Pressing that copy-link button gives you a link you can give to anyone to share the file. This is especially useful for Form level help in Medidata Rave. Pasting this URL into the Form help preceded by %% means that when published anyone clicking the help button in Rave will open the file from TrialGrid without needing a TrialGrid login. An example file name might be:
%%https://beta.trialgrid.io/public/TEST_STUDY/FIXYA/CompletionGuidelines/FIXYA_CRF_Guidelines.pdf
Note that users with this link can't see any other content in TrialGrid.
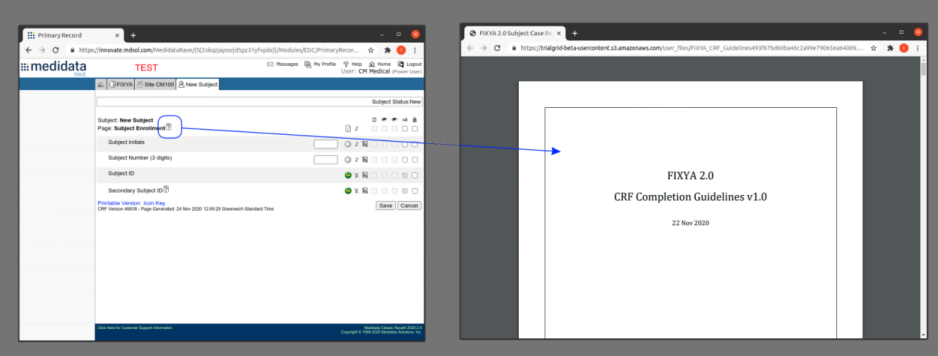
The benefit of using TrialGrid to host CRF Completion Guidelines in this way is that the TrialGrid Files area is controlled by permissions so only users with rights to modify files and permission to make files public can change them. It is also completely self-service, allowing the team to easily change the guidelines just by switching the file in the TrialGrid system without changing anything in the Rave study design.
Summary
CRF Completion Guidelines are an important support to sites but creating them and making them available online can be a challenge. In this article we showed how to use the HelpText %% feature of Rave to link to Guidelines hosted by Medidata Rave or by other systems. We also discussed the features available in Rave for creating and hosting CRF Completion Guidelines and the features in TrialGrid that support these activities.
If you are interested in better ways to generate and host CRF Completion Guidelines for Medidata Rave studies please get in touch!