Medidata recently celebrated its 20 year anniversary,
an amazing milestone, congratulations to all our friends and colleagues at Medidata!
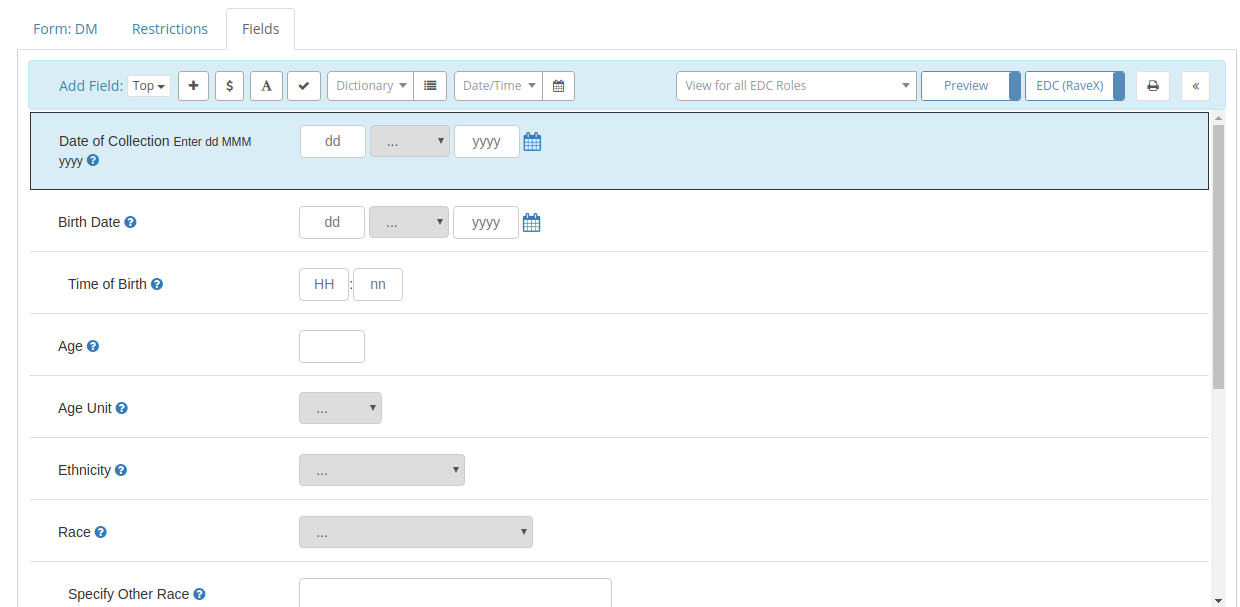
Rave Architect, the Form and Edit Check design part of Rave, isn't quite 20 years old and in fact it still looks remarkably
fresh, but nearly 20 years after it was first created, the needs of its users have grown. Some Rave installs have been
running for more than a decade, accumulating hundreds of studies and reaching a scale that, I'm sure, would surprise
the original designers of Architect.
That scale brings with it organizational challenges. Teams of 2 or 3 study builders in a single location have become
departments of fifty or more study builders spread across different continents and timezones. Which projects are
active? Which projects belong to which therapeutic areas? Which are the Phase III or Phase IV projects? Rave Architect
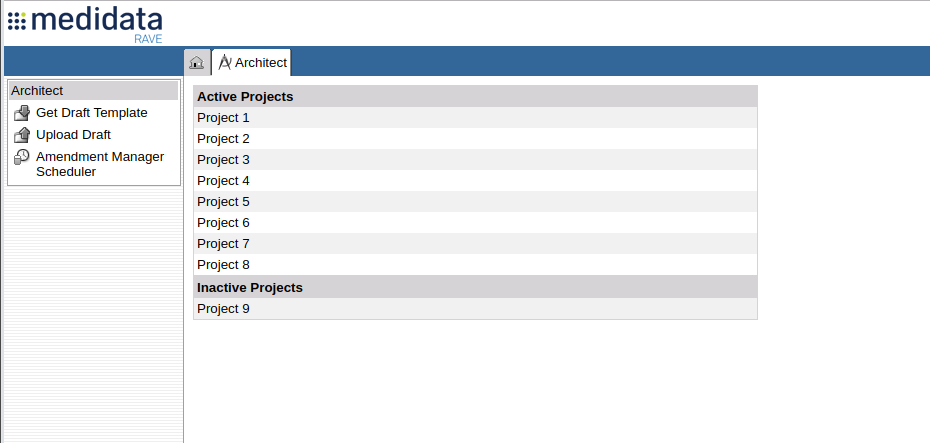
doesn't have the ability to capture that Metadata. The Project listing in Architect gives you two useful
attributes: The name of the Project and whether or not the Project is Active.
This isn't a criticism of Architect; It's a tool designed to help you with the work of building and publishing EDC
studies. It isn't a project planning tool, a team coordination platform or a Metadata repository. As an organization
that uses Architect you need to provide those things.
As a result Organizations have tools for Project planning, technical document management, specifications management,
UAT findings tracking, programming review and many more - some of them use commercial software but many of them are
home-grown using spreadsheets and shared drives.
Our goal at TrialGrid is to provide a single, integrated environment for the nuts-and-bolts work of the study build but
also the tracking activities that go on around it. As every Clinical Programmer knows, the job isn't done when you
create a Form or an Edit Check. You also need to update a system or a spreadsheet to indicate that you've done that
work and that it's ready to be reviewed by a technical reviewer, a standards manager, a tester or a manager. Life
would be so much easier if there was a function in the study build tool that would perform that step.
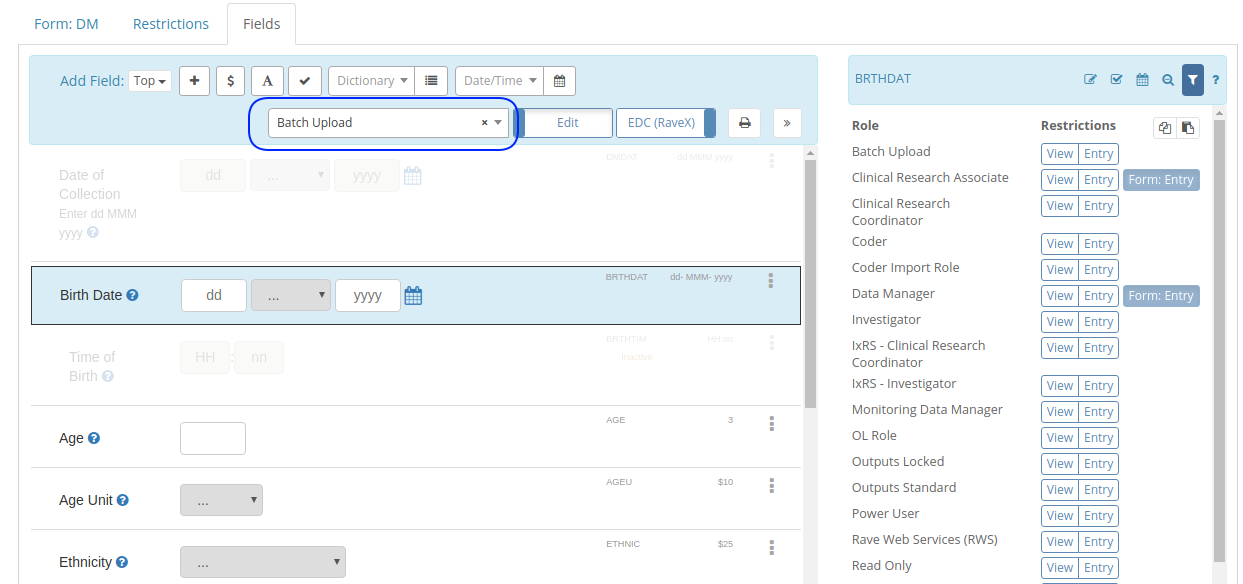
That's why in TrialGrid we provide Labels that can be applied to any study design object to signal a workflow state
(e.g. Ready for Review) or to provide informational tags (e.g. IxRS Integration) and Custom Metadata that can be
applied to Forms and Fields in the study design to capture additional information (e.g. Form Standards Version,
SDTM Annotation, E2B field relation, SDV tier).
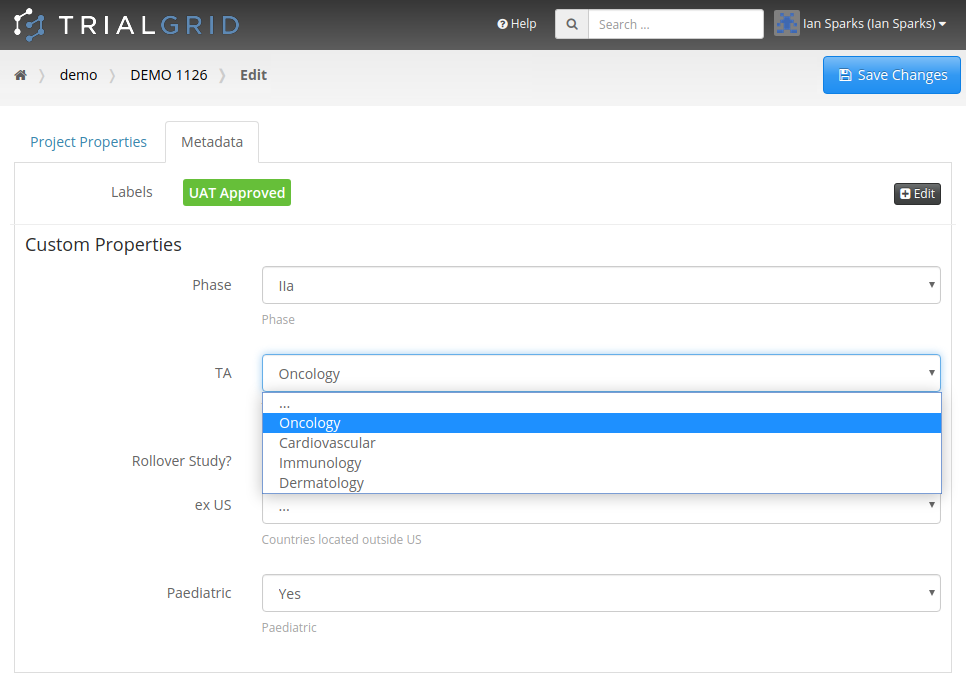
And this week we added the ability to apply labels and custom Metadata to Projects and to Drafts. Allowing you to
get a better overview of your Projects and to filter or search the list:
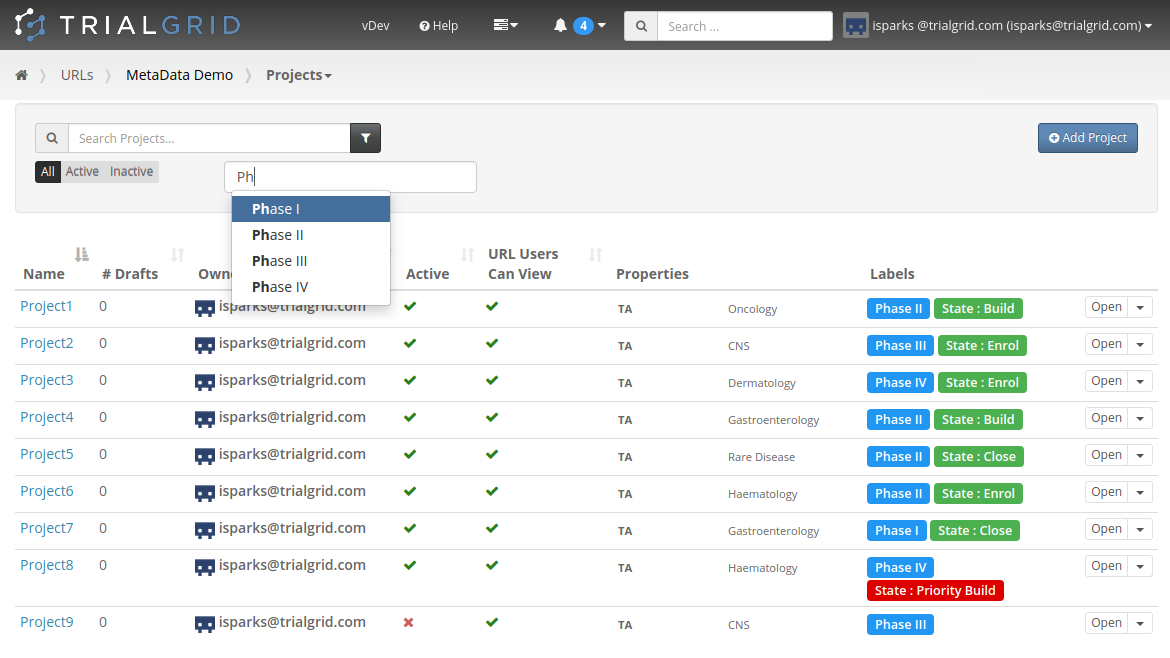
Just like Labels and Metadata on other object types, you get to choose the names and colors of Labels and the
additional Metadata you want to capture. If it's Metadata related to study design objects such as Forms, Fields or the
Draft itself then it is automatically exported to and imported from ALS files. This is useful if your source of
ALS files is not Rave Architect but some kind of Metadata Repository - now some of that useful Metadata can be
transferred along with the study design where it's helpful for Clinical Programmers.
We have further plans for this kind of metadata. Stay tuned!
If you would like to get future updates about this and other new features in TrialGrid, please use the subscribe button
above to subscribe to our newsletter.